Spatial Omics – How Creating a High-Resolution Tumor Blueprint Improves Cancer Immunotherapy Outcomes
Cancer immunotherapy has revolutionized oncology, offering the possibility of long-term control or even complete eradication of disease in some patients. Among these therapies, immune checkpoint inhibitors (ICIs) have achieved the greatest success in the treatment of solid tumors, dramatically improving outcomes across various cancer types.
However, the reality remains that a substantial proportion of patients either do not respond to ICIs or experience serious adverse effects. This unpredictability highlights a critical unmet need, a better predictive tool and a deeper mechanistic understanding of how these therapies work and why they sometimes fail.
This is where spatial omics technologies are emerging as game changers. By providing insights into the spatial organization of cells and molecules within the tumor microenvironment, spatial omics promise to enhance our understanding of cancer biology and lead to more personalized, effective treatments.
In this article, we’ll explore how spatial omics help address the current challenges in immunotherapy, offering a more precise, patient-centric approach to cancer treatment.
Introduction to Spatial Omics?
Spatial omics is a cutting-edge technology that maps positional relationships between cells within a tissue. Consider for example, tumor tissue, composed of five major cell types – tumor epithelial cells, many different immune cells, stromal cells, endothelial cells and normal epithelial cells. Spatial mapping identifies the location of each and every cell-type, and how they are organized with respect to each other.
One can generate genomics and proteomics data on top of the spatial data to create a readout of each cell. The readouts can be gene expression (spatial transcriptomics), protein expression (spatial proteomics) and so on.
One can think of spatial omics like creating a high-resolution map of a city:
- Instead of streets and buildings, you see cancer cells, immune cells, blood vessels, and other key cell types present in the tissue.
- You can see how buildings are connected to each other – through a bridge or a walkway akin to how cells are connected to each other in a tissue – one cell connected directly to another cell or via a blood vessel
- You can zoom in on neighborhoods (specific tumor regions) or even individual “houses” (single cells) to understand what’s happening. One can discern communication between cells and identify the route of information exchange. If the cells are away from each other, communication will be through diffusible factors, if they are in proximity, communication can happen through cell-cell interactions
- Finally, we can identify changes in the behavior of cells because of information exchange. One can find changes happening to immune cells, say T cells, when they are present near healthy epithelial cells, vs when they are in close proximity to tumor epithelial cells.
This high-resolution mapping of tumors reveals the intrinsic heterogeneity of the tissue, which is often highly variable from one area to another. Variability comes not only from the cellular composition but also how cells are organized with respect to each other.
Key differences between spatial omics and other omics platforms
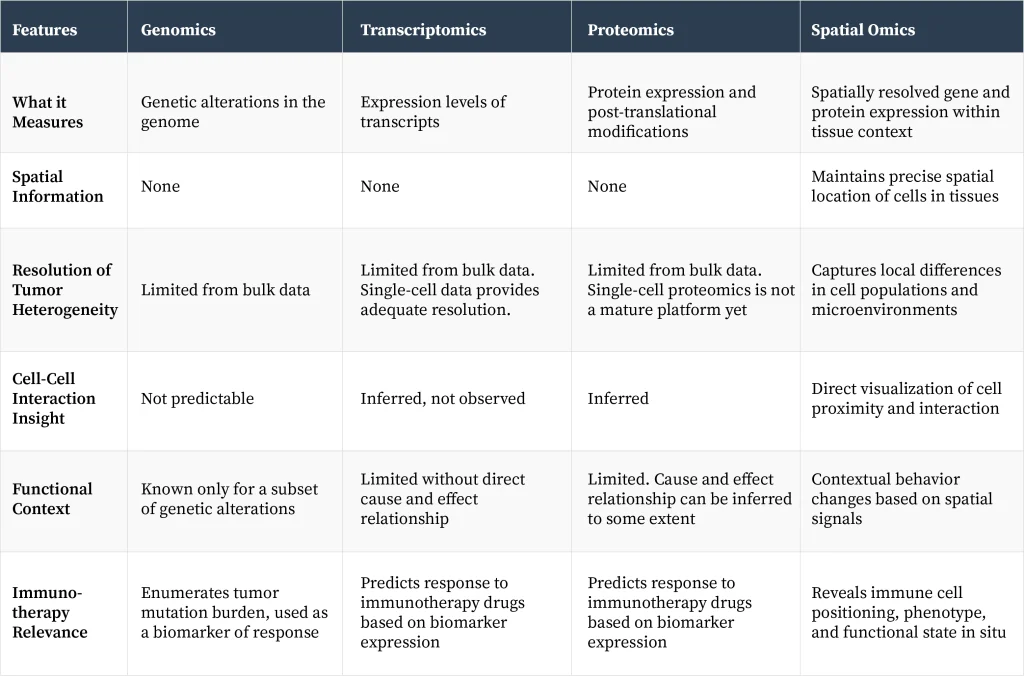
Understanding these differences can reveal why some therapies work in certain patients but not in others, why some patients’ tumors spread, while for others the tumor is localized? These insights help clinicians to manage diseases more efficiently, and drug developers use this knowledge to develop novel therapies.
Spatial Omics Has the Potential to Make Cancer Immunotherapy More Effective
Cancer immunotherapy relies on the body’s own immune cells to attack and eliminate tumors. Therefore, prior knowledge of how immune cells are organized in different regions of tumor tissue and what is the composition of immune cells in different regions of the tumor is crucial for cancer immunotherapy to work. Spatial omics has addressed some of the most pressing challenges in cancer immunotherapy:
- Predictive Insights: Detailed spatial maps can quantitate the presence of different immune cells in the tumor microenvironment. It also uncovers functional patterns in these cells, which can inform whether the tumor will respond to treatment or remain unresponsive. Such knowledge can help to select patients who will benefit from the therapy. Given that cancer immunotherapy induces toxicity, unresponsive patients can be spared from adverse effects of the drug.
- Identification of New Therapeutic Targets: By revealing immune cell dynamics and tumor-immune interactions, spatial omics technologies can identify novel points of intervention. For example, in tumors that are immune deserts – meaning lack of infiltration of immune cells, a therapy to improve immune cell infiltration will make cancer immunotherapy drugs work more effectively.
- Tracking Tumor Evolution: Spatial analysis enables the study of tumor subclones and their distinct behaviors within different regions of the tumor mass. Sub clonal populations of cells harbor unique genetic alterations responsible for drug resistance and tumor relapse.
- Characterization of Immune Cell Behavior: Clonal expansion of B and T cells in different regions of the tumor and the identity of their receptors – B and T cell receptors, reveal the presence of unique immunogenic tumor antigens, which can contribute to the development of next-generation cancer vaccines and cell therapies.
- Beyond Conventional Biomarkers: Spatial technologies can reveal critical biological changes in the tumor microenvironment, including metabolic shifts and structural DNA alterations, that traditional diagnostics are likely to miss.
Collectively, these insights open to new refined therapeutic strategies, reduce unnecessary side effects, and ultimately improve patient outcomes and their quality of life.
Challenges in Clinical Implementation
Despite its transformative potential, integrating spatial omics technologies into routine clinical practice presents significant obstacles:
- Data Complexity: Spatial omics generates data that can produce clinically actionable insights through advanced computational infrastructure and analytical frameworks, not readily integrable with clinical protocols.
- Sample Handling Requirements: While some platforms are compatible with standard formalin-fixed, paraffin-embedded (FFPE) tissues, others require fresh frozen specimens, necessitating adjustments to current sample collection protocols.
- Cost and Turnaround Time: Spatial analyses remain expensive and time-intensive, limiting their feasibility for large-scale clinical deployment at present.
- Operational Complexity: Effective use of spatial technologies demands close collaboration among multidisciplinary teams, including clinicians, laboratory scientists, and data analysts — a logistical challenge for many institutions.
- Sampling Limitations: As with all tissue-based diagnostics, there is an inherent risk that a small biopsy may not fully capture the heterogeneity of the entire tumor.
- Clinical Usability: Hospitals and clinics require diagnostic tests that are rapid, standardized, and easily interpretable, whereas spatial analyses currently involve complex procedures and specialized expertise.
- Understanding Systemic Effects: Immunotherapy exerts effects throughout the body, yet spatial technologies predominantly focus on the tumor site. Integration with systemic data, such as from blood or lymph nodes is necessary for a comprehensive interpretation.
- Causality vs. Correlation: While spatial mapping can identify associations, understanding the underlying biological mechanisms requires perturbational studies, including the development of new model systems like patient-derived organoids, which may not fully recapitulate the intrinsic features of a tumor.
What Can We Expect in the Foreseeable Future?
The applications of next-generation technologies to solve problems in human health, such as genome sequencing, identification of biomarkers by high throughput mass spectrometry and analysis of tumor microenvironment demonstrate that complex innovations can, over time, become integral to routine care if they are clinically impactful.
For spatial omics technology, the immediate opportunity lies in targeted application: Deploying high-resolution spatial analyses in selected patient groups where detailed insights can have direct clinical benefit, for example, a treatment strategy when first, second, and third-line therapies have failed. Faster clinical adoption and widespread use will come when the technology gets further refined to enhance speed, reduce cost, and simplify workflows.